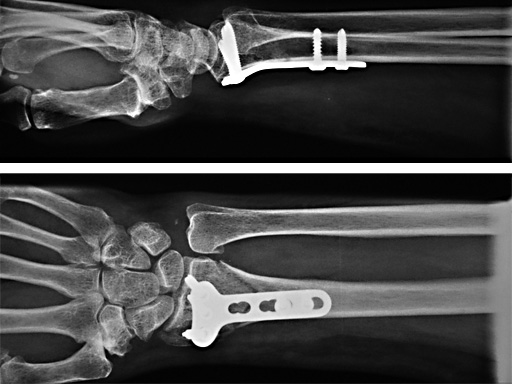
2.4 mm LCP Distal Radius Plate
Clinical studies
One of the key objectives of AOCID is to organize and conduct clinical studies that provide evidence regarding the clinical benefit of AO methods and implants. The gold standard study design to achieve this is a randomized clinical trial (RCT). We therefore considered this design for the LCP distal radius study right from the start.
While simple on paper, the process of randomization met with some practical obstacles. Which treatment would be optimal to compare the LCP with? For which indication? Would surgeons be prepared to randomize patients? In fact, we could not find a consensus on the best control treatment, since the new LCP plate is based on a new concept and does not replace an existing system on a one by one basis. An RCT was judged inappropriate, in particular because surgeons were convinced that the LCP will provide a better outcome for the patient and therefore were not willing to use the conventional system any longer.
The best compromise was to conduct an observational study where the treatment decision remains in the hand of the surgeons. This design allows documentation of the range of indications for treatment with the LCP, and both clinical and radiological outcomes, after plating of the distal radius. By collecting data from a large number of cases, we will identify different cohorts (e.g. patients treated with different plates) that will be compared.
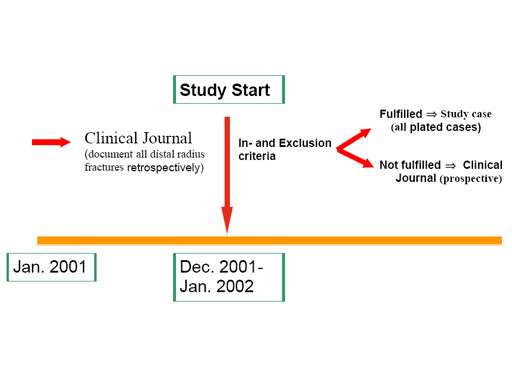
The LCP Distal radius study includes three prospective studies and one retrospective study.
- Conservative distal radius study in the UK documenting all distal radius fractures, and all types of treatment (majority of fractures treated conservatively).
- LCP 3.5 mm distal radius study, involving centers mainly using the 3.5 mm LCP system (10 centers).
- LCP 2.4 mm distal radius study, focused on the 2.4mm LCP system (6 centers).
- Retrospective study documenting the treatment of all distal radius fractures in the past year (before the LCP plate was available). The same clinical journal form is used, with no follow-up visits, and no collection of X-ray images (7 centers).
We have two types of questionnaires for the prospective studies, one for all plated cases (any type of plate and fulfilling inclusion and exclusion criteria), including follow-up forms up to two years post-operatively, and a shorter one for all other cases (making the clinical journal).
A schematic overview of the LCP 3.5 mm and 2.4mm study, combined with the retrospective study is shown in the figure below. The same inclusion and exclusion criteria, as well as clinical (including the Gartland and Werley, the DASH and the SF-36 scoring systems) and radiological (centrally evaluated) outcome criteria are used in the different prospective studies. While follow-up is implemented for all cases in the "Conservative" distal radius study, only plated cases are followed in the two other prospective studies.
Special attention will be given to the expected advantages of the new LCP concept as well as to maintenance of reduction, the additional use of bone grafting, and the learning curve. The studies are controlled in order allow a comparison of the LCP systems to other conventional plating systems and conservative treatment of distal radius fractures. The retrospective part of the study (documenting the situation before the introduction of the LCP system) will show whether any shift in indication from e.g. external fixator to the LCP system has occurred and in order to define a control group accordingly for future studies.
The different studies were started at the end of 2001.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO Foundation”
The brands and labels “approved by AO Technical Commission” and “approved by AO Foundation”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.