T-PAL
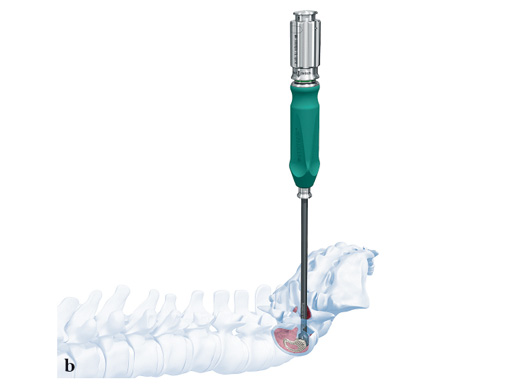
The new T-PAL system is an innovative minimally invasive surgical cage system designed to replace lumbar intervertebral discs and facilitate fusion of the adjacent vertebral bodies at vertebral levels L2/S1 in a transforaminal approach.
Spinal fusion has traditionally been effective in treating low back pain and instability associated with spinal stenosis, degenerative disc disease, and deformity. While many studies indicate that transforaminal and posterior fusions with instrumentation result in improved fusion rates, the traditional open transforaminal and posterior procedure can result in severe patient morbidity, muscle tissue damage, open incisions, large areas of bony resection, excessive blood loss, and longer operating room time.
In traditional TLIF procedures one major disadvantage is the difficulty in placing the implant in the correct anterior position. This often requires frequent hammering and many changes of the insertion instrument position. Not only has this been found to be too time-consuming by many surgeons, but the numerous passes in and out of the disc space can also result in an increased risk of nerve damage. Finally, it has not been possible to position the trial implant where the final implant will be, making implant selection more challenging.
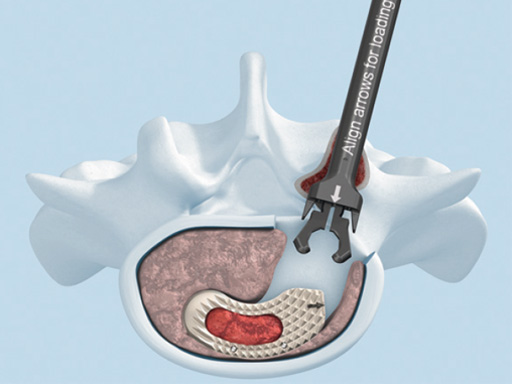
The development of T-PAL is a result of surgeons' feedback on the currently existing solutions for transforaminal lumbar fusion, in particular the request for improved instrumentation. It offers one main instrument the applicatorwhich can be used for both the trial positioning and the controlled placement of the implant in minimally invasive procedures. This instrument also features a security button to prevent premature implant disengagement.
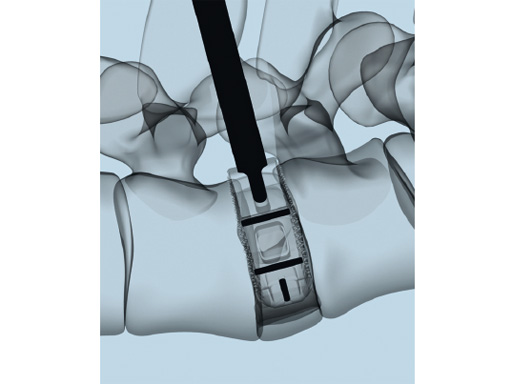
The key new feature of T-PAL is the guidance rails on upper and lower surfaces of both the trial and final implants which guide the implant into the final position. This self-guidance feature places the implant exactly where the surgeon desires it in the disc space and consequently saves time and eliminates the need to constantly readjust the insertion angle. Unlike traditional rigid trials, the T-PAL trial implant pivots, allowing it to be placed in the same location as the implant.
The kidney-shaped implants are manufactured globally from PEEK and most recently titanium (US only); they are available in two footprints (28 x 10 mm and 32 x 12 mm) and 11 heights (717 mm in 1 mm increments). With the exception of the shortest implant (7 mm high) all of these offer a 5 lordotic angle.
Indications outside the US are lumbar and lumbosacral pathologies in which segmental spondylodesis is indicated, for example:
- Degenerative disc diseases and spinal instabilities
- Revision procedures for postdiscectomy syndrome
- Pseudarthrosis
- Degenerative spondylolisthesis
- Isthmic spondylolisthesis
It is important to note that T-PAL requires additional posterior fixation.
Indications for the T-PAL spacer within the US are for patients with degenerative disc disease at one or two contiguous levels from L2S1 whose condition requires the use of interbody fusion combined with supplemental fixation. The interior of the T-PAL spacer should be packed with autogenous bone graft (ie, autograft).
Degenerative disc disease is defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies. These patients should be skeletally mature and have had six months of nonoperative treatment.
The T-PAL spacer is intended for use with supplemental fixation, eg, TSLP, ATB, Antegra, Synthes USS (including Matrix, USS Small Stature, Click'X, Pangea, USS Polyaxial, USS Iliosacral, and ClampFix).
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO Foundation”
The brands and labels “approved by AO Technical Commission” and “approved by AO Foundation”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.