Prodisc-C Vivo
Those that continue to have significant pain may have symptomatic cervical disc disease, which is defined as neck or arm (radicular) pain and/or a functional/neurological deficit with at least one of the following conditions confirmed by imaging (CT, MRI, or x-rays):
- Herniated nucleus pulposus
- Spondylosis (defined by the presence of osteophytes)
- Loss of disc height.
The traditional approach to relieving such pain is a standard decompression and fusion at the symptomatic levels. While this technique has demonstrated good patient outcomes in terms of pain reduction, there may be other unintended consequences that result from altering the natural biomechanics of the cervical spine [1].
Cervical Total Disc Replacement (TDR) may provide the solution. Decompression and restoration of disc height can be achieved as normal to alleviate pain. In addition, the preservation of the preoperative range of motion and restoration of biomechanical stability may reduce the incidence of adjacent-segment degeneration [2].
The Prodisc-C Vivo is the next evolution of the cervical TDR. Building on the success of the pre-existing Prodisc-C family, the new design keeps the features that have proved so clinically advantageous, while incorporating new features that reduce the required remodelling and simplify the overall technique.
Prodisc-C Vivo maintains the following features at the core of the already successful Prodisc-C family of implants:
- Fixed center of rotation that resists shear forces and enables controlled motion
- Ball-and-socket articulation using the proven combination of CoCrMo/UHMWPE
- Titanium alloy endplates that reduce the MRI artefacts.
Additionally, Prodisc-C Vivo offers the following new benefits:
- Primary fixation with spikes
- Anatomical implant design with convex cranial endplate
- Simple surgical technique with two main steps, trialing and implant insertion.
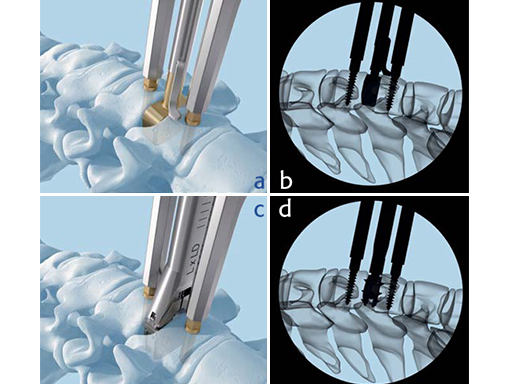
Because of the anatomical, keel-less design of the Prodisc-C Vivo, vertebral body preparation is not required, which simplifies the surgery and enables a less traumatic implantation. Furthermore, the endplates of the vertebral bodies generally do not require remodelling to accommodate the implant. This reduces OR time and leaves the bone intact, minimizing the potential risk of postoperative implant subsidence.
Again, because the endplates do not require remodelling the instrumentation of the Prodisc-C Vivo is done in two simple steps: trialing and implant insertion.
References
- Gore DR, Sepic SB, Gardner GM (1987) Neck pain: a long-term follow-up of 205 patients. Spine 12(1):15.
- Murrey D, Janssen M, et al (2009) Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine 9(4):275286.
a-b) Trial to determine implant size. b-c) Implant insertion.
Case: Man with neck and arm pain
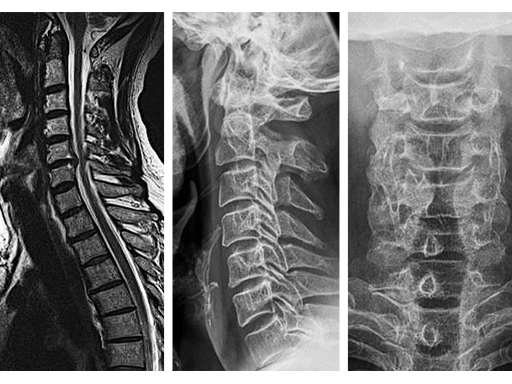
A 50-yr-old man presented with a 4 month history of neck and arm pain on the right side. Repeated trials of conservative treatment, including imageguided injections, had not led to a significant relief of symptoms. Clinical examination showed a C6 radiculopathy on the right side with pain, hypoaesthesia, and a slight biceps weakness.
The MRI (Fig 1a) showed a disk herniation C5/6 medial and paramedian right-sided. The preop xrays (Fig.1b-c) showed a slight narrowing of the disc space at C5/6. The segment was still mobile.
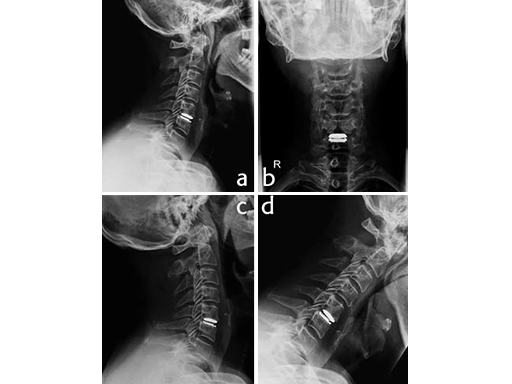
A microsurgical discectomy, decompression and implantation of a Prodisc-C Vivo implant was performed (Fig. 2a-b).
The early and 6 month outcome was excellent. The segment showed preserved mobility (Fig. 2c extension and Fig. 2d flexion). The patient returned to work after two weeks and returned to normal activities.
Cervical total disc replacement - Prodisc-C Vivo
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO Foundation”
The brands and labels “approved by AO Technical Commission” and “approved by AO Foundation”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.