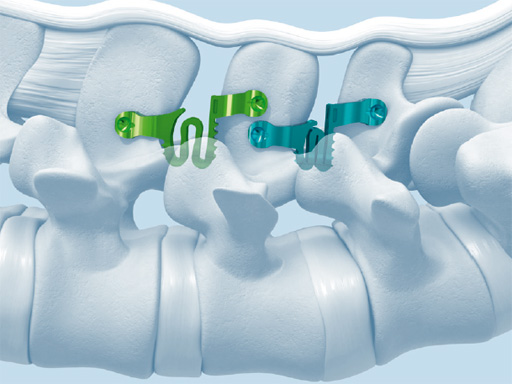
StenoFix
Stenofix is an interspinous implant used after decompressive surgery. The device is intended for use as a space holder between the spinous processes for one or two lumbar levels L1S1. The resulting effects on the posterior elements are preservation of the foraminal height, reduction of stress on the facet joints and reduction of pressure on the posterior annulus.
The W-shaped spring allows for dampening of high axial loads. The implant is designed with divergent wings to ensure implant insertion. For an optimized anatomical fit the shape of the cranial radius of the implant fits into the natural concavity of the superior spinous process. Further, the wings facilitate double-level implantation.
The stenofix implants are available in sizes 816 mm in 2 mm increments. Construction material is titanium alloy and supplied sterile. Each implant is color coded along with trial implants of the same size and shape.
Fig 1 Staggered wings facilitate double-level implantation.
Fig 2ab A 66-year-old man with L3/L4, implant size 10.
A 56 year-old-man had low-back pain for 10 years, with 2 years of sciatica radiating into the left leg. He had no sensory or motor deficiency.
Nonoperative treatment failed completely. Surgery included decompression of level L4/5 and implantation of an interspinous stenofix L4/5 device size 12. He had a normal postoperative course with rapid decrease of pain.
Case provided by Andreas Korge, Mnchen, Germany
Fig 1 X-ray shows dynamic extension 3 months postoperatively.
Fig 2 Intraoperative view with original stenofix implant in place.
Cases provided by Andreas Korge, Mnchen, Germany
Clinical case 1
A 66-year-old man with L3/L4, implant size 10.
A 56 year-old-man had low-back pain for 10 years, with 2 years of sciatica radiating into the left leg. He had no sensory or motor deficiency.
Nonoperative treatment failed completely. Surgery included decompression of level L4/5 and implantation of an interspinous stenofix L4/5 device size 12. He had a normal postoperative course with rapid decrease of pain.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO Foundation”
The brands and labels “approved by AO Technical Commission” and “approved by AO Foundation”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.