ARI research pearls
Breakthrough antibiotic-releasing hydrogel enables single-stage revision surgery after infection
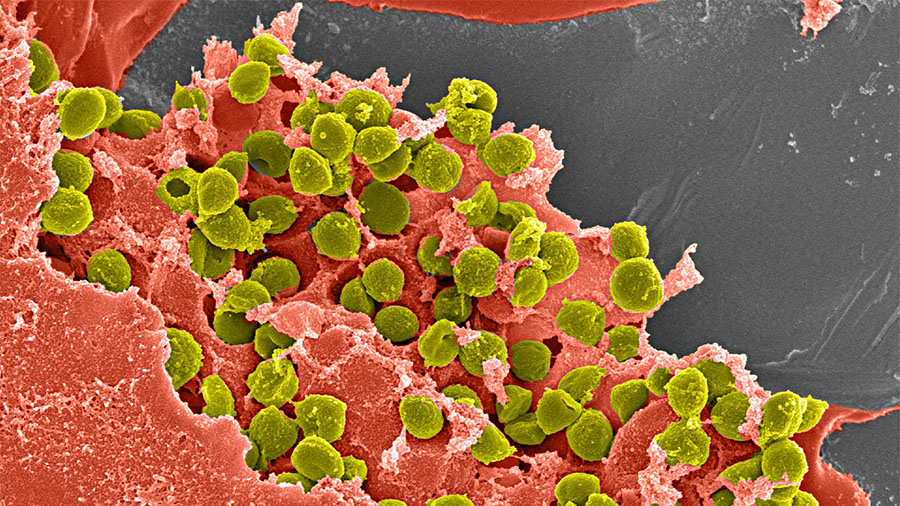
Though orthopedic infection is an uncommon problem, affected patients often face a succession of revision surgeries as well as potential functional limitations. These are precisely the patients whose care—and lives—could be transformed by a breakthrough antibiotic-releasing hydrogel pioneered by the AO Research Institute Davos (ARI) as part of the AO Trauma Clinical Priority Program on Bone Infection.
“Years of research have led to this breakthrough. Since the gel with an antibiotic is classed as a drug and not an implant, obtaining regulatory approval will be extremely expensive. This can only be undertaken with strong industrial support, despite the massive effect this technology can have on treating and preventing infections and improving patients’ lives,” said ARI Director Prof Geoff Richards.
According to ARI Focus Area Leader for Biomaterials Matteo D’Este, the innovation takes a two-pronged approach to orthopedic infection. It can be injected locally to prevent infection in high-risk patients such as those with severe open fractures, the elderly, and those with diabetes or circulatory problems, or it can be used as a treatment that eradicates confirmed infection and enables single-stage revision surgery.
“In cases where orthopedic implants become infected, the patient faces a big ordeal: removal of the implant, placement of a spacer to maintain dead space during systemic antibiotic therapy that may last months, and only once the infection is cleared will the patient receive their replacement fixation device and healing can once again commence,” D’Este said. “Our antibiotic-releasing gel can potentially be applied in every situation where bone infection is an issue: both as a preventative and as a support to therapy. Importantly, due its nature as a soft hydrogel, it can be applied in hard-to-reach areas that are not even visible to the human eye, and it degrades without leaving any residual material in the tissue.”
The concept nicely illustrates the interdisciplinarity of ARI, with its expertise in a wide range of scientific specialties including biomedical materials and infection biology. The work has also benefitted from the expertise and facilities available at ARI’s Preclinical Services program, which has built up many years of experience in testing antimicrobial biomaterials in complex small- and large- animal models of bone infection.
An example of ARI’s collective expertise on this topic was recently published in a peer-reviewed scientific article demonstrating that the antibiotic-loaded hydrogel is as effective as a one-stage procedure compared to the current gold standard: antibiotic-loaded bone cement. Importantly, the hydrogel-treated group could receive the definitive replacement nail in the same surgery; the hydrogel was simply injected around the nail. The spacer group of course, did not have a fully functional, metal implant. This shows that the hydrogel can maintain a high treatment success rate, but also offers a simpler and more direct course of surgical treatment. The findings of that study, “Single-stage revision of MRSA orthopedic device-related infection with an antibiotic-loaded hydrogel,” were published in the Journal of Orthopedic Research in December 2020.
Looking forward, the goal is to translate this technology from the laboratory to the clinic. A number of challenges remain, not least of which are the regulatory hurdles required to prove the efficacy of such an intervention in human patients. Through the AO Innovation translation Center, the team has been supported by Andrea Montali of the AO development incubator and has received funding to begin the translation of this concept. A key benefit of ARI’s antibiotic-loaded hydrogel is that—unlike antibiotic-coated implants like plates and femoral nails—only one such gel would need to be approved in order for it to be applied to every implant. This should ensure that, once available, the hydrogel may benefit a wide range of patients.
ARI Focus Area Leader Infection Biology Fintan Moriarty said the antibiotic-loaded hydrogel underscores the AO’s mission of promoting excellence in patient care.
“Fewer infections and higher treatment success rates will all contribute to improved patient care,” Moriarty said. “Any reduction in infection is very welcome. Sometimes, these patients keep going back for repeated revision surgeries, to the point that so much bone is debrided that there are significant defects, or they give up and take antibiotics for the rest of their lives. So, the hydrogel represents a life-changing improvement in treatment—and the potential for years of life lived without disability.”

