Update from the AO Smart Digital Solutions Task Force 2023
Wearable-based tracking of functional recovery
Building on the identified needs from the AO’s Wearable Technology survey, the AO Technical Commission's Smart Digital Solutions Task Force (SDSTF) conducted a feasibility study to determine the value of wearable technology in assessing the postinjury functional recovery―the bring your own device (BYOD) study. Results of this study have been published in an AO Special Issue of Medicina [1].
Back to normal? ―Bring your own device
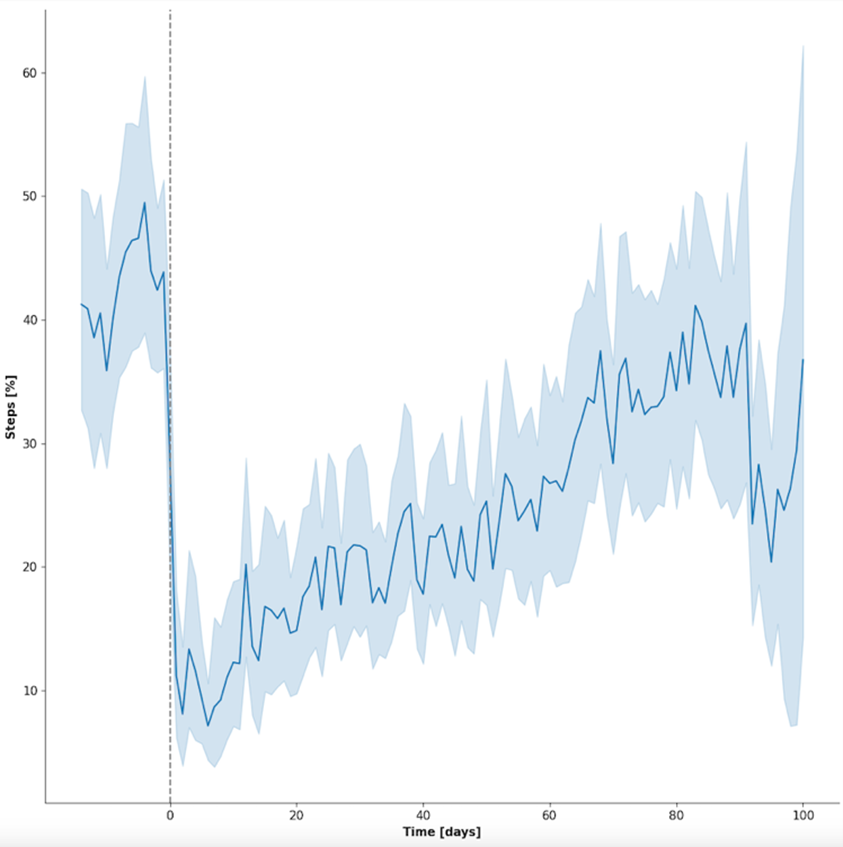
For this feasibility study, patients with any acute musculoskeletal injury were included that already had a personal wearable system (smartphone, or wrist worn device) prior to their injury (BYOD). By collecting the step counts for each day as a device agnostic parameter and normalizing them to the one time maximum for each patient, a clear recovery trajectory can be seen from the otherwise highly diverse patient group (Fig 1). This allows for a visualization of the functional recovery in relation to the pre-injury, individual function as determined by the daily step count.
Fig 1 The patient recovery process from 14 days preinjury to up to 100 days postinjury as tracked by the daily step count is shown. The left graph reveals the daily step average for all patients normalized to the one time maximum for each patient (dark blue line) and the 95% confidence interval (light blue shade). The X-axis shows the time in days (0 = day of injury); Y-axis, the normalized step activity in percentage. (Courtesy of Braun BJ et al. Injury. 2023 Nov 30;55(2):111254.)
One step further
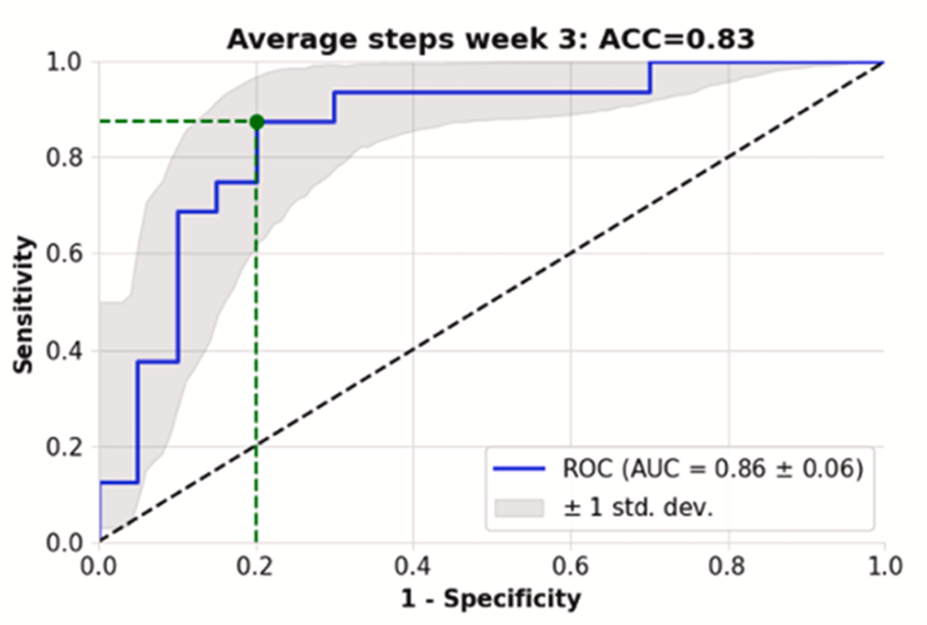
The feasibility study highlights the main benefits of this new approach, in that the otherwise largely existing blind spot of preinjury functional status becomes readily assessable. Not only can the functional recovery be visualized with this approach but also by further analyzing it with a logistic regression model, predictions about the functional recovery can be made early during the treatment course (Fig 2). This has the potential to detect patients at risk for a prolonged healing course at an earlier timepoint. The results of this preliminary machine learning approach to assess wearable data have been published [2].
Fig 2 Receiver operating characteristic curve demonstrating the predictive model’s performance to detect at least 50% functional recovery of the preinjury activity state by week 6, based on the step data up until week 3. X axis shows 1-specificity; Y axis, sensitivity. The threshold was chosen to maximize (sensitivity + specificity) and is indicated in green. Accuracy was calculated based on this threshold. (Courtesy of Braun BJ et al. Injury. 2023 Nov 30;55(2):111254.)
Challenges on the way to the further adoption of BYOD and wearables
The current analysis shows the feasibility of the approach in a clinical setting. Limited patient number and inclusion of a broad range of injuries does not yet allow for a definition of recovery boundaries. More extremity and injury-specific data is required to provide aftercare recommendations based on this technique. Also, our understanding of baseline wearable data (ie, step count) in relation to physical function needs clarification, while the potential of different wearable systems from other medical fields must be explored.
Resolving these new challenges will require dedicated research efforts. The group is already conducting several projects aimed at better understanding the value of different wearable systems, the relation of wearable data, and objective physical function (ie, VO2 max), while looking to increasing the BYOD patient database with simplified data-harvesting solutions. Further updates will follow.
References
- Braun BJ, Histing T, Menger MM, et al. “Bring Your Own Device”—A new approach to wearable outcome assessment in trauma. Medicina (Kaunas). 2023 Feb 19;59(2):403.
- Braun BJ, Histing T, Menger MM, et al. Wearable activity data can predict functional recovery after musculoskeletal injury: feasibility of a machine learning approach. Injury. 2023 Nov 30:111254.