AO ITC: The innovative core of the AO's global network
2020 saw the realization of a new approach to foster innovation within the AO by the creation of a new AO innovation hub—the AO Innovation Translation Center (AO ITC), which comprises four business units, the AO Technical Commission, Clinical Operations, Clinical Science, and Technology Transfer. The AO ITC brings together AO teams engaged in innovation, streamlines the process, and makes innovation translation at the AO more efficient and agile.
Innovation translation at the AO encompasses the conception, strategic evaluation, and development of clinical solutions in collaboration with industrial partners. It includes proof-of-concept work with innovators, valorization of new technologies and techniques and clinical evidence creation to prove the added value of new solutions. Strategic investments in intellectual property, technology, and companies are also part of AO ITC's mandate. At the center of all innovation is the AO's global network of surgeons and other healthcare providers who contribute ideas and expert guidance at all stages of development. Throughout the process, the AO ITC provides the expertise and resources to foster an environment where ideas are translated into clinical solutions addressing the needs of modern healthcare in a rapidly evolving environment.
Structure of the AO ITC
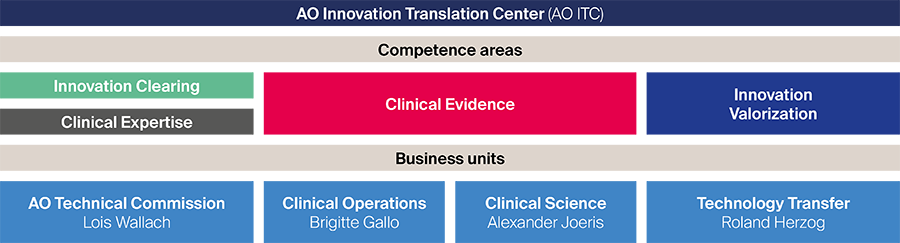
Four complementary competence areas (innovation clearing, clinical expertise, clinical evidence, and innovation valorization) are embedded in a process-driven matrix under four business units (Fig 1) which are responsible for the realization of innovation projects and serving internal and external partners. The creation of the AO ITC allows quicker evaluation of new concepts from innovators, harmonizes budgeting processes, and reduces administrative hurdles.
Fig 1 Organizational structure of the new AO Innovation Translation Center.
Key activities according to competence areas
Clinical expertise
The AO ITC places expert clinical guidance from leading surgeons at the center of the innovation translation process, covering all relevant areas of trauma, spine, craniomaxillofacial, veterinary, and other surgical fields as required. Dedicated groups of key opinion leaders define unmet clinical needs and identify the best solutions to address these needs in line with the overall AO strategy. The development of clinical solutions is executed by close collaboration between expert surgeons and selected industrial partners.
Surgeons engaged in the AO ITC also support the AO's endeavor to provide education for new surgical techniques through the creation of educational materials and may participate as course faculty. Approval by the AO Technical Commission serves as a prerequisite for new technologies to be taught in surgical courses organized by the AO's clinical divisions.
Fig 2a–b he AO Technical Commission at work: medical experts provide clinical guidance throughout the development process for new solutions.
Innovation clearing
The expert surgeons monitor major innovation activities within their areas of expertise, define the most feasible methods to realize new concepts, and determine the evidence creation strategy for each project at its inception. To ensure high quality solutions to clinical problems, innovation clearing at the AO ITC follows a dedicated development process including the definition of clinical needs, assessment of new concepts, priority setting, implant and instrument design, preclinical and clinical evaluation, and eventual approval. Regular communication between the AO Technical Commission and the AO's industrial partners allows effective sharing of opinions and alignment of priorities to realize new collaborative projects. Following market release, the clinical performance of new solutions is reported in Expert Symposia, in which groups of surgeons of all levels of experience meet regionally and review clinical cases, both good and bad, in which new technology is used.
Fig 3a–f Expert group meetings conducted by the AO Technical Commission: sharing opinions and strategies for new collaborative projects.
Clinical evidence
All AO ITC projects are firmly grounded in clinical needs and are compatible with the AO's philosophy of evidence-based treatment development. Each new solution must provide measurable benefits for patients and physicians and improve the healthcare landscape. Aside from the exploration and validation of new technologies during product development, with the launch of the new European medical device regulation, high-quality clinical evidence becomes ever more essential in the development and post-market surveillance of medical devices.
The AO ITC's clinical evidence competence is based on the expertise of the two in-house clinical research units, Clinical Operations and Clinical Science, which operate in compliance with a Process Management System based on the ICH GCP (Good Clinical Practice) and ISO 14155. At the outset of a clinical project, the Clinical Science team collaborates closely with the lead surgeon (and the representatives of an external partner, if applicable) to generate an appropriate study protocol with optimal design and statistical considerations so as to lay a firm foundation for high- quality clinical data. The Clinical Operations team ensures the faithful execution of study protocols through compliance with the state-of-the-art operational standards and applicable ethical guidelines to select, train, and monitor participating clinics. At the end of a clinical study, the Clinical Science team conducts data analysis, reporting, and publication in peer-reviewed journals to ensure dissemination of the knowledge. Close collaboration of these two units and their teams of specialists ensures that the AO ITC can support both internal and external partners with their regulatory and clinical research needs. Early involvement of Clinical Science and Clinical Operations ensures the delivery of high-quality clinical evidence with sound scientific methodology.
Aside from offering services in database management, statistical analyses, and medical journalism, Clinical Science and Clinical Operations also contribute toward the development of young surgeons into future clinical researchers. Team members of these two units host clinical research fellows in the Dübendorf office and conduct the GCP course under AO PEER's auspices.
Fig 4a-d Staff from Clinical Operations and Clinical Science at work, supporting evidence-based solution development.
Innovation valorization
Innovation valorization at the AO ITC encompasses business intelligence gathering, idea consolidation, and market research. On behalf of the AO Foundation Board, the Technology Transfer team performs these tasks and manages dedicated AO innovation funds, consisting currently of the Development Incubator and Strategy Fund, as well as projects derived from these funds. Headed by Michael Schütz, the AO Technology Transfer Board guides all innovation valorization activities and includes experts in areas such as business development, start-up companies, financing, and medical device research and marketing.
The Technology Transfer team aims to support inventors in product development through a well-structured pathway that transforms ideas from benchtop to bedside. Funding, knowledge leadership, expert insight, proof-of-concept, clinical validation, and valorization are all parts of this pathway, which ultimately leads to commercially viable products. Along this pathway, the team also helps to identify, attract, and negotiate with suitable commercial partners to ensure the eventual commercialization of the projects through either licensing or selling of assets derived from them.
In areas deemed strategically important to the AO, the AO ITC may propose investment in partners needed to achieve the AO mission. These strategic goals may include innovation leadership in orthopedic technology to address unmet clinical needs, and leadership in education solutions for the global orthopedics community.
The Technology Transfer unit publishes calls for proposals for the Strategy Fund and Development Incubator regularly. Declined proposals are evaluated in cooperation with experts within the AO Technical Commission to make sure that real clinical needs are covered. To see examples of current Technology Transfer projects, refer to the articles on the Biphasic Plate and the Robotics System.
Fig 5a-b AO Technology Transfer supporting the proof-of-concept development of an innovative small external microfixator.
Synergy at work
Since its launch in March 2020, the AO ITC has improved the efficiency of the innovation workflow within the AO. New concepts for surgical solutions are evaluated by expert surgeons and are allocated to the appropriate development pathway, whether with the AO's primary industrial partner DePuy Synthes (DPS), the Development Incubator, or with other carefully selected industrial partners. Importantly, evidence creation is planned and initiated at the outset of every new project. This workflow crosses the previous boundaries of the AO Technical Commission, AO Clinical Investigation and Documentation, and the Development Incubator.
Since 2015, the AO Technical Commission has been able to realize development projects with alternative industrial partners in the event of a project proposal being declined by DPS and following approval by the AO Foundation Board. Expertise from the Technology Transfer unit in the areas of market evaluation and business planning has been invaluable in the development of robust business cases for these "off-ramp" projects. Additionally, selected project proposals declined by DPS are currently under evaluation by the Technology Transfer Board for potential development via the Development Incubator pathway. During the evaluation process, key opinion leaders from the AO Technical Commission’s network provide expert insight to inform project shortlisting.
Voice of customer data is of increasing importance in the modern healthcare environment to support new product development. The creation of the AO ITC has enabled the AO Technical Commission to leverage the Clinical Evidence team's expertise in data gathering and analysis to create surveys for distribution to the surgeon network, both to evaluate market trends and clinical needs and to monitor the performance of newly launched products.
In keeping with the coming of the big data age, the AO ITC is the driving force behind the AO Global Data initiative. Transcending traditional registries, this initiative brings together advancements in computer science and the AO's leadership and integrity to incorporate clinical data from the AO’s global network into benchmarked data sets. These are relevant to not only researchers and clinicians but also to healthcare providers and the medical device industry. Only the AO and the AO's network of surgeons can offer such unique, unbiased, evidence-based information for trauma and musculoskeletal disorders. As a valued part of the AO network, our expert surgeons play a vital role in making the AO’s voice heard globally.
The creation of the AO ITC has opened new pathways and opportunities for the development of cutting-edge surgical solutions and strengthens the AO's position as the premier institution to improve patient outcomes in the treatment of trauma and musculoskeletal disorders.