3.5 LCP Distal Femoral Osteotomy Plates
Background
Patellar luxation is a common cause of pelvic limb lameness in dogs. Although patella luxation can occur because of trauma, most canine cases of patellar luxation occur because of abnormal femoral and tibial modeling during skeletal development. Traditional surgical correction of canine patellar luxation includes procedures such as sulcoplasty and tibial tuberosity transposition. These techniques are often insufficient in more severe cases resulting in recurrence of patellar luxation. Complex limb deformities involving both the femur and tibia (eg, distal femoral varus, femoral torsion, and a compensatory tibial valgus and/or torsion) typically explain these surgical failures. Distal femoral osteotomy/ostectomy (DFO) was developed to address distal femoral varus (with or without torsion) and has led to markedly improved clinical outcomes. Current implants for canine DFO are not anatomically specific to follow both the femoral procurvatum and condylar morphology, nor do they guide screw trajectory to avoid the femoral intercondylar notch and the femoral trochlea, especially with locking implants. As such, veterinary surgeons may encounter challenges with plate contouring and screw placement.
Plate Design
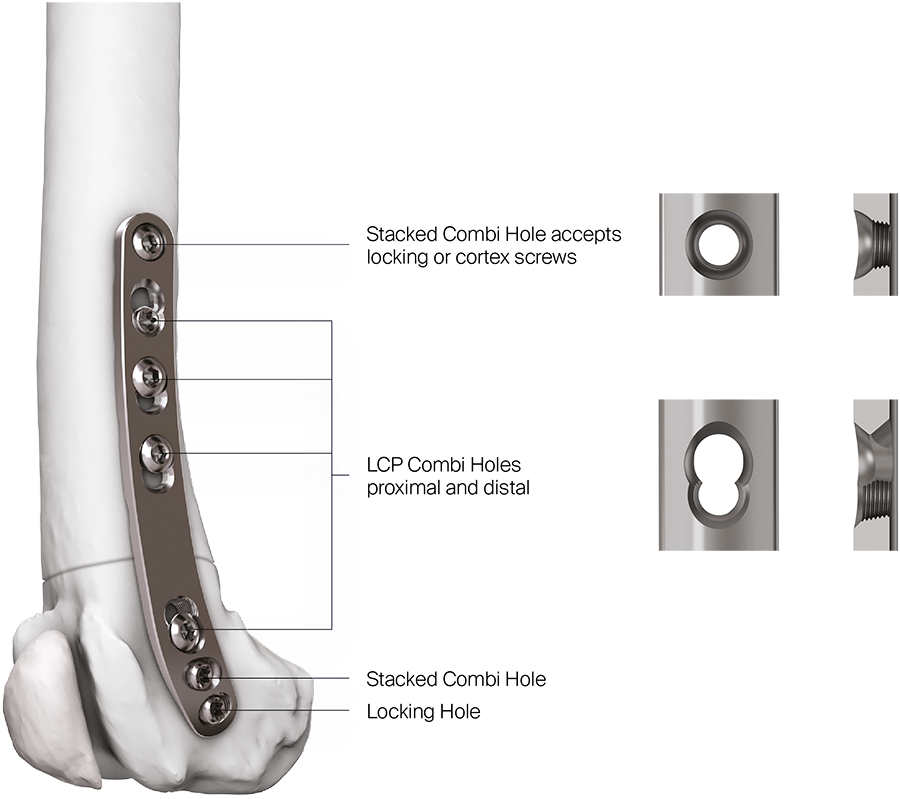
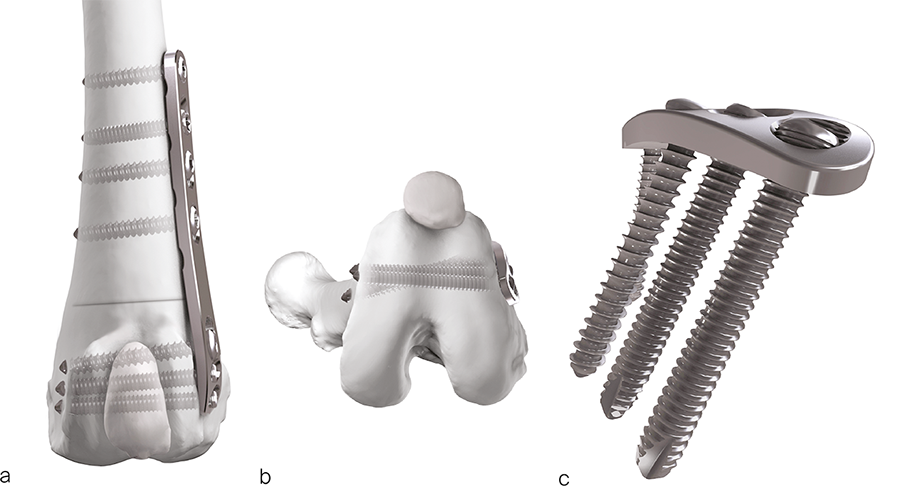
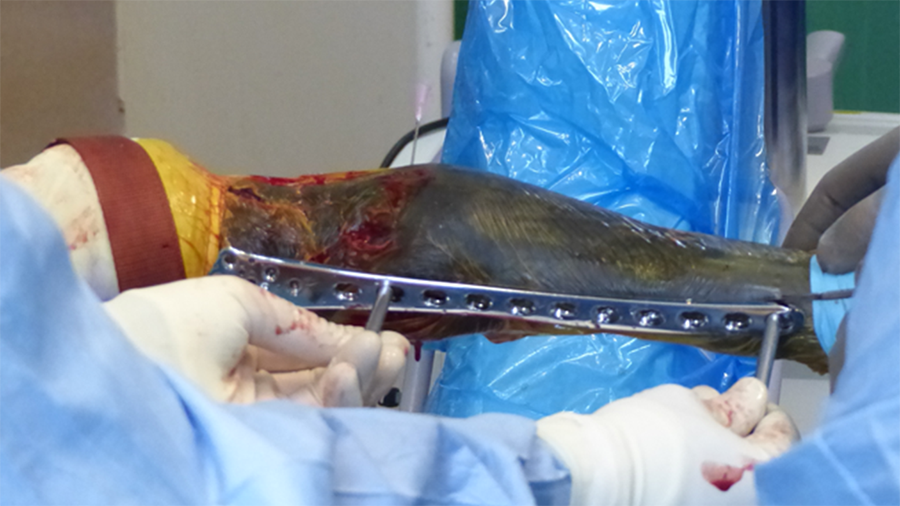
The recently approved 3.5 LCP DFO plate is indicated for treatment of distal femoral angular deformities and distal femoral fractures. The plate curvature was designed to accommodate procurvatum (anatomical curvature in the sagittal plane) and optimize screw trajectories into the widest portion of the femoral condyle while simultaneously avoiding the regions of the intercondylar notch and femoral trochlea in medium and large breed dogs. The plates are available in 7- or 8-hole lengths to account for variable femoral sizes encountered in medium and large canine patients. Additionally, the plates are specific for left and right limbs (Fig 1). They are compatible with both 3.5 mm locking and cortex screws (Fig 2). The trajectories of the three distal locking screws were designed to avoid the intercondylar notch, maximize screw purchase in the caudal portion of the femoral condyle, while also avoiding cranial screw placement so as to preserve bone for concurrent sulcoplasty (Fig 3). The tapered, curved design minimizes interference of the distal plate with both the patella and periarticular soft tissue. Click here for more detailed information about the 3.5 LCP DFO plate and its features.
Fig 1a–b The left (a) and right (b) 7-hole and 8-hole 3.5 LCP Distal Femoral Osteotomy Plates.
Fig 2 Distribution of LCP and stacked combi holes in the 3.5 LCP Distal Femoral Osteotomy Plate.
Fig 3a-b Screw trajectories on the distal end of the 3.5 LCP Distal Femoral Osteotomy Plate.
Clinical Cases
The following two cases (kindly provided by Michael Kowaleski, North Grafton, Mass, USA, and Erik Asimus, Toulouse, France) illustrate common canine femoral deformities associated with moderate to severe medial patella luxation and demonstrate application of the 3.5 LCP DFO Plate.
Case 1: Distal femoral osteotomy in a 4-year-old, female spayed mixed breed dog with medial patellar luxation
A 4-year- and 6-month-old spayed female mixed breed dog weighing 25.1 kg presented for chronic progressive left pelvic limb lameness. Orthopedic examination and preoperative radiographs revealed a grade 3/4 medial patellar luxation without concurrent cranial cruciate ligament rupture. A preoperative computed tomography (CT) was performed to screen for femoral varus and/or femoral torsion. Femoral varus was documented (anatomical Lateral Distal Femoral Angle [aLDFA] 104°, normal 92-96°). The femoral torsion angle was 26° and was like the unaffected contralateral limb and within the normal range. The planned correction was a 12° lateral closing wedge ostectomy, with concurrent sulcoplasty and tibial tuberosity transposition (Fig 4 ). Click here to read through the detailed surgical procedure.
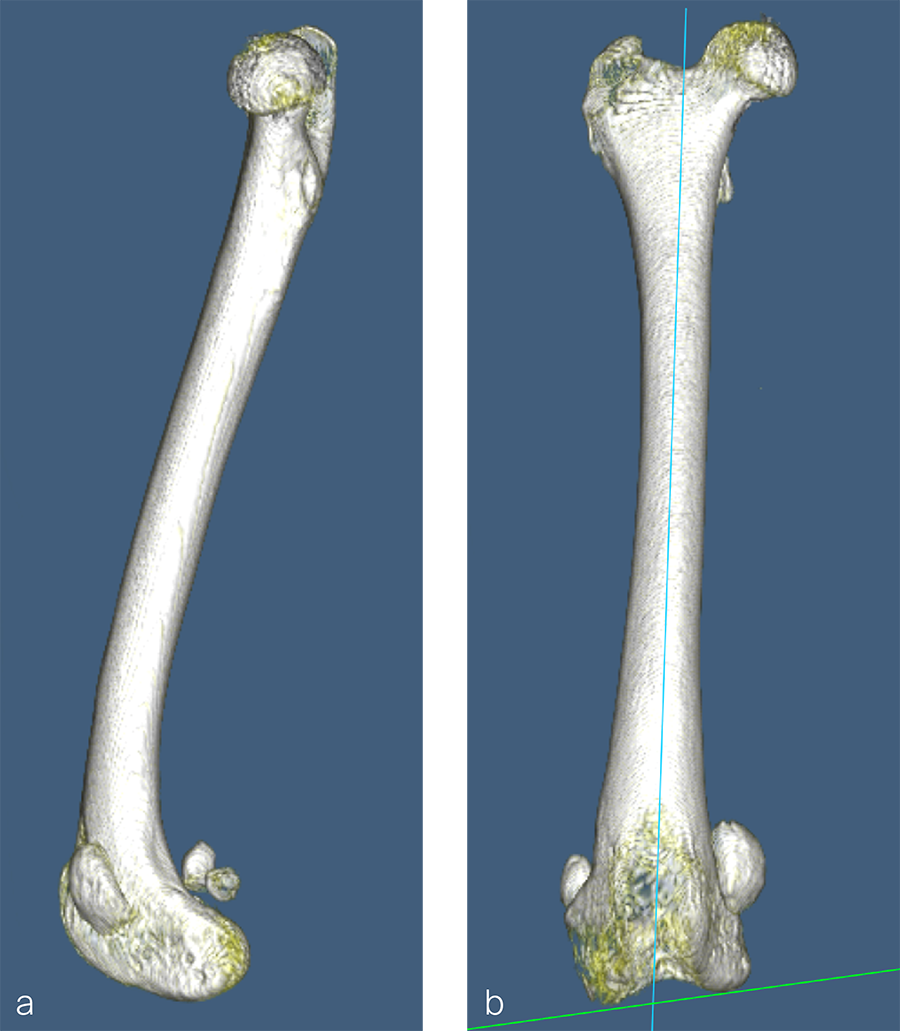
Fig 4a–c Preoperative CT 3D reconstruction of the left femur. a Mediolateral view illustrates anatomical anteversion of the femoral head and neck and procurvatum of the femur. b Craniocaudal view confirms distal femoral varus (anatomical Lateral Distal Femoral Angle, 104°). c Femoral long axis view shows anteversion angle (26°).
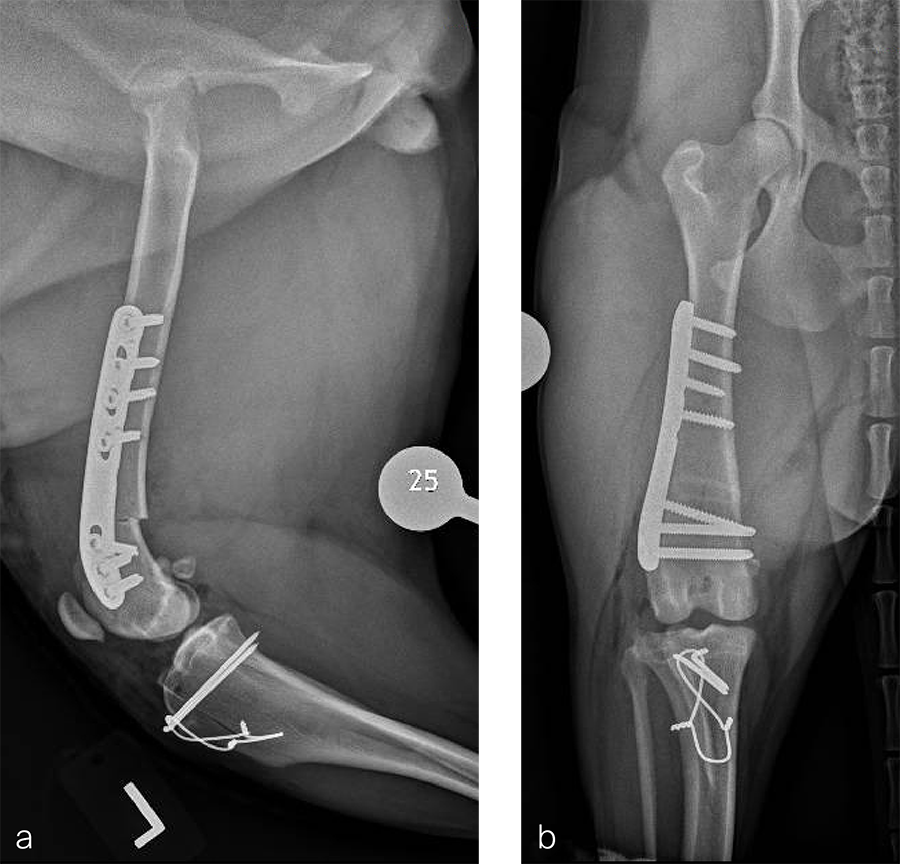
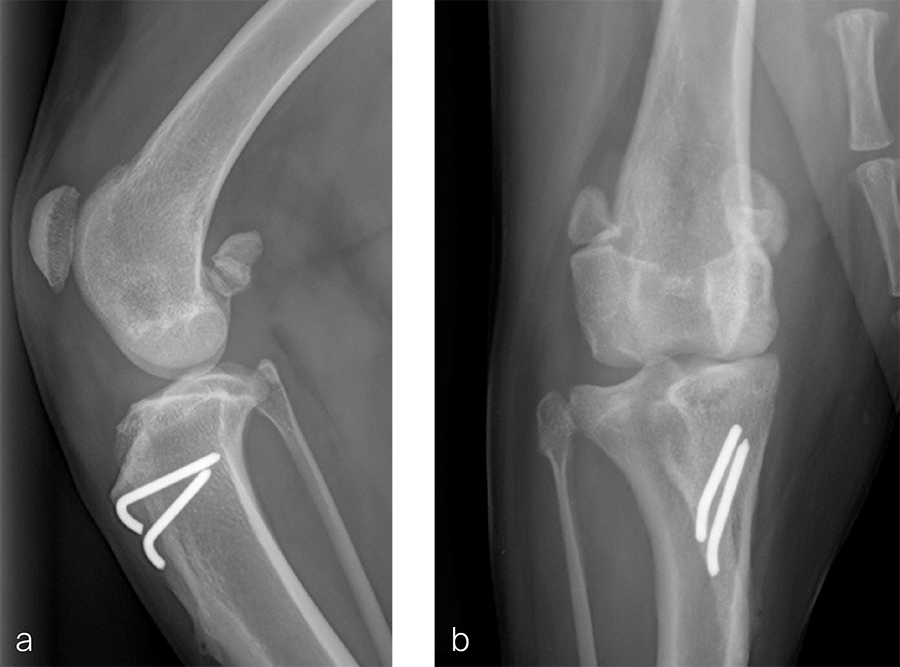
Fig 5a–b Postoperative radiographs demonstrating position of a left, 7-hole, 3.5 LCP DFO plate and associated tibial tuberosity transposition. a Medioateral view. Note that plate contour matches the normal distal femoral procurvatum and screws are positioned caudally away from the trochlea. This allows for an unimpeded sulcoplasty while maximizing screw purchase caudally. The compression applied with the plate has resulted in excellent apposition. The patella is visible within the trochlear groove. b Craniocaudal view. The plate contour matches the anatomical contour of the distal femoral condyle. The anatomical Lateral Distal Femoral Angle is reduced to 92°.
Fig 6a–b Follow up after eight weeks. Clinical signs have resolved, limb use is excellent, and stifle joint range of motion is normal without evidence of pain. a Mediolateral radiograph. The osteotomy has healed. Plate and screw position remain unchanged. The tibial tuberosity transposition is healing, and implants remain unchanged. The patella remains reduced within the trochlear groove. b Craniocaudal view. The osteotomy has healed and is no longer visible. Implants are stable and the patella is tracking normally.
Case 2: Distal femoral osteotomy in a 1-year- and 6-month- old spayed female Appenzell Cattle Dog
A 1.5 year old spayed female Appenzell Cattle Dog weighing 29 kg presented for a second opinion after two previous surgical procedures (Fig 7 and Fig 8) to address a traumatically induced medial patellar luxation. Orthopedic examination and preoperative radiographs revealed a grade 3/4 medial patellar luxation (Fig 8). Femoral varus was documented (aLDFA 104°; normal 92–96°) by CT scan (Fig 9 ). The planned correction was a 10° lateral closing wedge ostectomy, with concurrent sulcoplasty. Click here to read through the detailed surgical procedure.
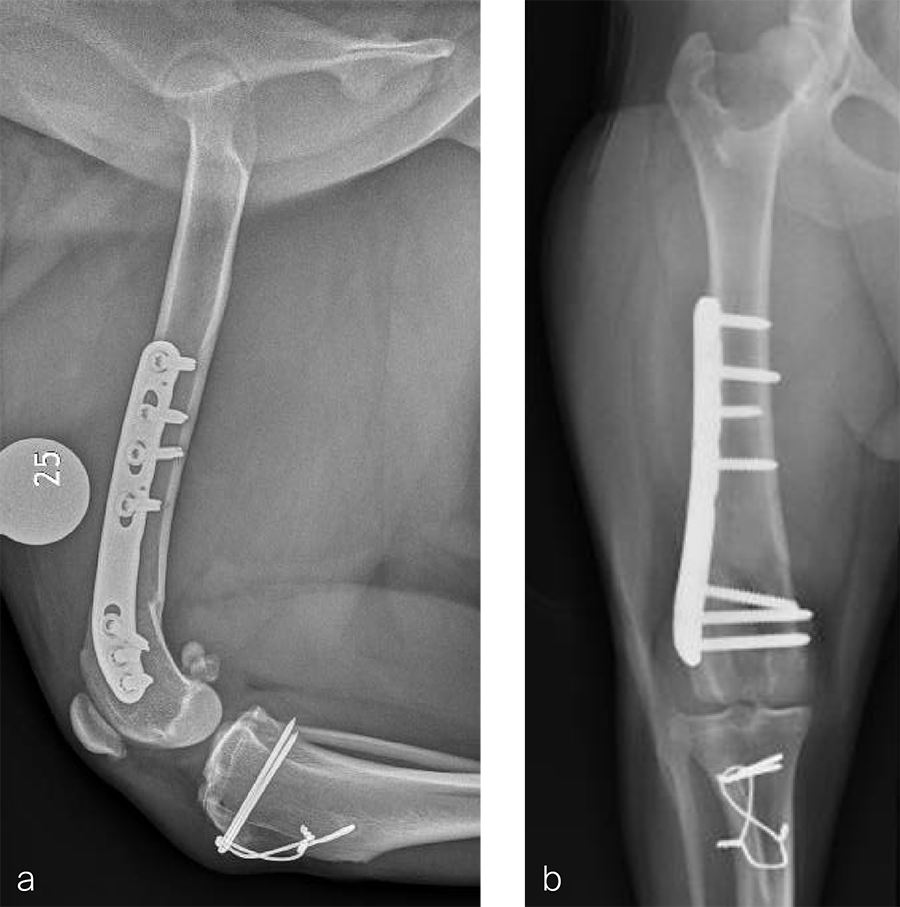
Fig 7a–b Initial surgery performed at 1 year and 6 months of age. The patellar luxation was treated with a lateral imbrication. No primary orthopedic procedures were performed. Radiographs were obtained six weeks postoperatively, and document persistence of the medial patellar luxation.
Fig 8a–b Revision surgery performed to address persistent patellar luxation. A tibial tuberosity transposition was performed to realign the insertion of the patellar tendon without addressing femoral deformity. Radiographs were obtained two months postoperatively. The patellar luxation persists despite transposition of the tibial tuberosity.
Fig 9a-b Preoperative CT 3D reconstruction of the right femur confirming distal femoral varus (anatomical Lateral Distal Femoral Angle was 104°). Planned correction was to perform a 10° lateral closing wedge ostectomy.
You might also be interested in
Veterinary Screw Targeting Clamp (STC)
A targeted screw placement system permits improved accuracy of screw insertion
LCP Distal Femur Plate for veterinary applications
To treat comminuted fractures of the proximal phalanx in horses with the goal of improving survival and reducing complications.
LCP Equine T-Plate 4.5
Designed to provide a single implant solution with multiple locking screw fixation in the epiphyseal segment as well as the ability to place locking screws throughout the length of the plate, to augment fixation in the soft, immature foal bone.
Cannulated Screw System CSS+
Improving cutting performance and facilitating accurate screw insertion.
Disclaimer:
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
The brands and labels "approved by AO Technical Commission" and "approved by AO Foundation", particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.